The Role of Chemomechanical Coupling in Skin and its Clinical Implications
Mechanical Forces in Skin
Internal and external mechanical forces naturally occur in skin on various magnitudes and time scales. It is known that such forces can actively affect skin mechanics, biology, and wound healing. There are even several therapeutic products on the market, such as tissue expanders and negative pressure wound therapy, which apply forces to skin for therapeutic purposes. However, it is not clear exactly what mechanisms are behind these effective mechanotherapies.
By improving our understanding of the types of forces needed to elicit a biological response, particularly in the main skin cell type - dermal fibroblasts, we can improve existing mechanotherapies, such as skin expansion, to reduce the duration of the treatment or improve its quality.
Inverse Poroelastic Behavior of Skin Leads to Chemomechanical Coupling
It has recently been discovered in our lab that stretching soft collagenous tissues, such as skin, leads to an efflux of water, thus increasing hydrostatic and osmotic pressure in the tissue. [1], [2] Such a coupling between stretch (mechanical stimulus) and osmotic pressure (chemical stimulus) offers one explanation of how mechanical forces affect skin biology and how they can be sensed by the dermal fibroblasts.
This has interesting implications in skin conditions where skin hydration levels, and therefore osmotic pressure, can be critical. For example, wound healing or symptoms of atopic dermatitis can be improved by increasing humidity, thus decreasing the osmotic pressure in the skin. [3], [4] We are interested in whether modulating skin tension is analogous to modulating skin hydration and whether skin stretch can be used therapeutically.
Dermal Fibroblast Response to Osmotic and Hydrostatic Pressure in vitro and ex vivo
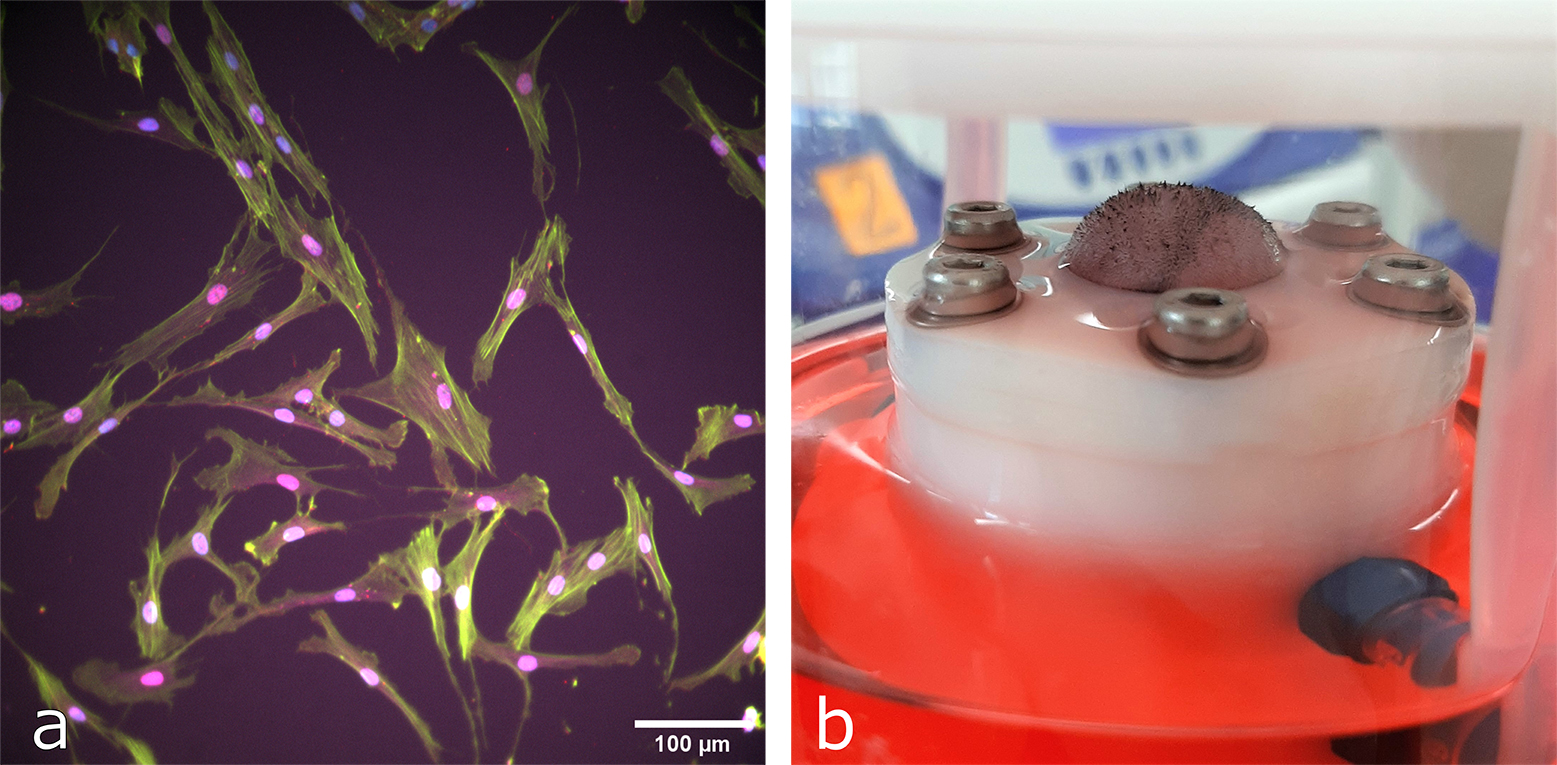
While several studies have shown isolated effects of modulating skin tension in rodent models [5], [6], none have studied the link between mechanical and osmotic stimuli. This project uses 2D and 3D in vitro cell models and bioreactors previously developed in the lab to study dermal fibroblast response to such stimuli on the gene expression level [7]. Furthermore, the project aims to compare how cells in whole murine skin (ex vivo) respond to osmotic and hydrostatic stimuli in comparison to mechanical stretch. (Figure 1).
Project Lead

Anastasiya Martyts
Collaborations
Werner Lab, Institute of Molecular Health Sciences, ETH Zurich
This project is part of the external page SKINTEGRITY.CH research initiative.
Funding
This project is supported by the Swiss National Science Foundation (grant no. 205321_179012).
[1] A. E. Ehret, K. Bircher, A. Stracuzzi, V. Marina, M. Zündel, and E. Mazza, “Inverse poroelasticity as a fundamental mechanism in biomechanics and mechanobiology,” Nat. Commun., vol. 8, no. 1, Dec. 2017, doi: 10.1038/s41467-017-00801-3.
[2] A. Wahlsten, M. Pensalfini, A. Stracuzzi, G. Restivo, R. Hopf, and E. Mazza, “On the compressibility and poroelasticity of human and murine skin,” Biomech. Model. Mechanobiol., vol. 18, pp. 1079–1093, 2019, doi: 10.1007/s10237-019-01129-1.
[3] K. Seltmann et al., “Humidity-regulated CLCA2 protects the epidermis from hyperosmotic stress,” Sci. Transl. Med., vol. 10, no. 440, May 2018, doi: 10.1126/scitranslmed.aao4650.
[4] J. P. E. Junker, R. A. Kamel, E. J. Caterson, and E. Eriksson, “Clinical impact upon wound healing and inflammation in moist, wet, and dry environments,” Adv. Wound Care, vol. 2, no. 7, pp. 348–356, Sep. 2013, doi: 10.1089/wound.2012.0412.
[5] S. Y. Chu et al., “Mechanical stretch induces hair regeneration through the alternative activation of macrophages,” Nat. Commun., vol. 10, no. 1, Dec. 2019, doi: 10.1038/s41467-019-09402-8.
[6] S. Aarabi et al., “Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis,” FASEB J., vol. 21, no. 12, pp. 3250–3261, Oct. 2007, doi: 10.1096/fj.07-8218com.
[7] A. Wahlsten, D. Rütsche, T. Biedermann, E. Reichmann, and E. Mazza, “Mechanical stimulation induces rapid fibroblast proliferation and accelerates the maturation of human skin substitutes,” Biomaterials.