Conductive Skin
The project of Conductive Skin aims to give a long and healthy life in patients with terminal stage heart failure (HF) that are treated with mechanical circulatory support devices (e.g., VADs). VADs feature a pump whose life saving function depends on a large power driveline that connects with batteries and controller outside the human body. The driveline injures human skin and establishes a chronic wound that becomes vulnerable to infection (DLI) [1,4]. DLI is the leading cause for readmission lowering the survival rate, impeding normal lifestyle and significantly increasing the cost of therapy in VADs.
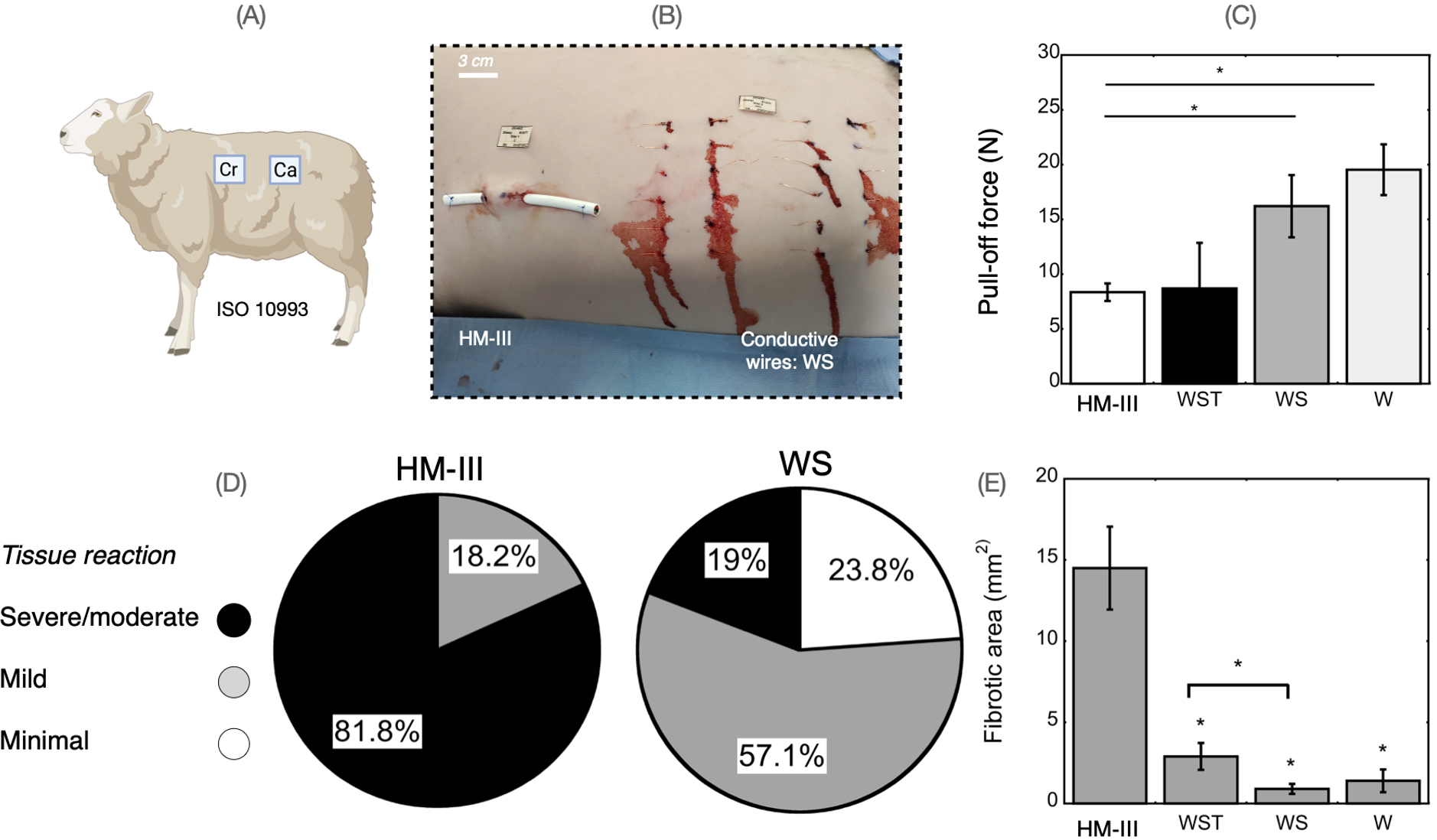
Our team has been leading targeted research and product development tasks to develop new drivelines that combat DLI. The project emerged from the collaboration of the ETH Zürich and the German Heart Center Berlin, involving world experts in biomechanics and VADs. The solution of Conductive Skin features adjustable biomechanics to prevent skin injury and safeguard the benefits of VAD therapy. We developed i) biocompatible wires with small diameter, low flexural strain, and select surface topography to enhance skin integration (Figure 1) and ii) a power transfer device that protects the barrier function of the skin.
Sponsors & Supporters
- Innosuisse: Innovation project of Conductive Skin
- Innosuisse: Initial coaching
- ETH Foundation: ETH Pioneer Fellowship
- National Strategic Initiatives: ETHeart, Zurich Heart & Skintegrity
Collaborations
- Dr. Nikola Cesarovic and Prof. Dr. Volkmar Falk (German Heart Center Berlin, Berlin, Germany & Department of Health Sciences and Technology, ETH Zurich)
- Julius Kaemmel and Prof. Dr. Christoph Starck (German Heart Center Berlin, Berlin, Germany)
[1] Andreas P. Kourouklis, Julius Kaemmel, Xi Wu, Miguel Baños, Astrid Chanfon, Simone de Brot, Aldo Ferrari, Nikola Cesarovic, Volkmar Falk, Edoardo Mazza, Transdermal wires for improved integration in vivo, Biomaterials Advances, 2023; external page https://doi.org/10.1016/j.bioadv.2023.213568
[2] Andreas P. Kourouklis, Adam Wahlsten, Alberto Stracuzzi, Anastasiya Martyts, Lorenza Garau Paganella, Celine Labouesse, Dunja Al-Nuaimi, Costanza Giampietro, Alexander E. Ehret, Mark W. Tibbitt, Edoardo Mazza, Control of hydrostatic pressure and osmotic stress in 3D cell culture for mechanobiological studies, Biomaterials Advances, 2023; external page https://doi.org/10.1016/j.bioadv.2022.213241
[3] PATENT; Fabrication method of an elastomer with topographical structure formed by the breath figure technique; Aldo Ferrari, Edoardo Mazza, Raoul Hopf, Andreas P. Kourouklis, Xi Wu; 2022; external page https://worldwide.espacenet.com/patent/search/family/073740176/publication/WO2022106290A1?q=pn%3DWO2022106290A1
[4] Andreas P. Kourouklis, Julius Kaemmel, Xi Wu, Evgenij Potapov, Nikola Cesarovic, Aldo Ferrari, Christoph Starck, Volkmar Falk, Edoardo Mazza, Systems of conductive skin for power transfer in clinical applications, Eur Biophys J Review, 2021; external page https://doi.org/10.1007/s00249-021-01568-8
[5] Xi Wu, Silvia Moimas, Raoul Hopf, Costanza Giampietro, Andreas P. Kourouklis, Volkmar Falk, Edoardo Mazza, Aldo Ferrari, A free-form patterning method enabling endothelialization under dynamic flow, Biomaterials, 2021; external page https://doi.org/10.1016/j.biomaterials.2021.120816
